Cn Lewi Dot Diagram
By putting the two electrons together on the same side we emphasize the fact that these two electrons are both in the 1s subshell.

Cn lewi dot diagram. A lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds. The lewis structure for li is li with one dot to the right of the element. This is the common. There arent enough valence electrons available for each atom to obtain an octet without sharing more than one pair.
For example the lewis electron dot diagram for hydrogen is simply. The most dangerous cyanides are hydrogen cyanide hcn and salts derived from it such as potassium cyanide kcn and sodium cyanide nacn among others. I also go over the hybridization shape and bond angle. I quickly take you through how to draw the lewis structure of cn cyanideion.
The number of dots equals the number of valence electrons in the atom. Lewis structures also known as lewis dot diagrams lewis dot formulas lewis dot structures electron dot structures or lewis electron dot structures leds are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. It is highly poisonous. A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
This is the common. Therefore cn has a triple bond between the carbon and oxygen atom. For example the lewis electron dot diagram for hydrogen is simply because the side is not important the lewis electron dot diagram could also be drawn as follows. 70 more lewis dot structures.
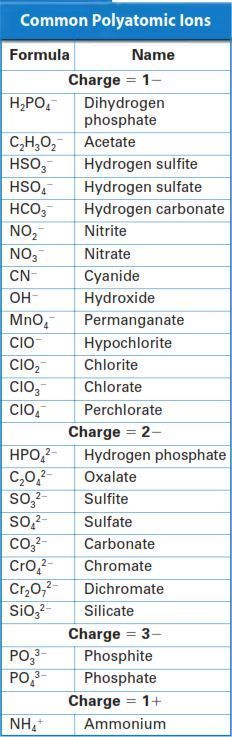
Lewis dot diagrams h h h h c c c o h h c h h h h c4h9oh lewis dot diagrams lewis dot diagrams are useful for visualizing both ionic and covalent bonds an oxygen atom. Cn is a negative ion called an anion. Consist of a carbon atom triple bonded to a nitrogen atom. Many cyanide containing compounds are highly toxic but some are not.
For the cn lewis structure there are a total of 10 valence electrons available. These dots are arranged to the right and left and above and below the symbol with no more than two dots on a side. Because the side is not important the lewis electron dot diagram could also be drawn as follows. The electron dot diagram for helium with two valence electrons is as follows.
By putting the two electrons together on the same side we emphasize the fact that these two electrons are both in the 1s subshell. A step by step explanation of how to draw the lewis structure for cn cyanide ion. There should be brackets around the final lewis structure with a 1 charge on the outside.